Swiss Garnier Genexiaa
Swiss Garnier Genexiaa
Swiss Garnier was established in the year 2004. The hi-tech manufacturing facilities were setup in Himachal Pradesh and Sikkim. During the short period, Swiss Garnier has achieved various accolades like Emerging India Award, Super SME Award and Business Excellence Award. Swiss Garnier has been accredited with WHO GMP and various countries' regulatory approvals. Swiss Garnier enjoys an envious position as one of the top 3 leading P2P manufacturers in India.
Swiss Garnier was established in the year 2004. The hi-tech manufacturing facilities were setup in Himachal Pradesh and Sikkim. During the short period, Swiss Garnier has achieved various accolades like Emerging India Award, Super SME Award and Business Excellence Award. Swiss Garnier has been accredited with WHO GMP and various countries' regulatory approvals. Swiss Garnier enjoys an envious position as one of the top 3 leading P2P manufacturers in India.
Company is establishing one more world class formulation facility at sikkim to add more valuable assets to this project looking out for deserving talents under various function as mentioned.
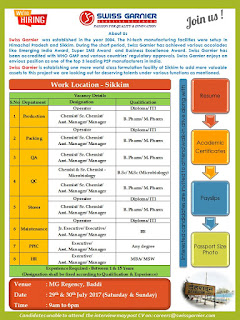
Work location - sikkim
Vacancy Detais;
- Production
- Packing
- QA
- QC
- Stores
- Maintenance
- PPIc
- HR
Interview Details :
Venue: MG Regency, Baddi,
Date: 29th & 30th July 2017 (Saturday & Sunday)
Time: 9am to 6 pm
For more details click on below image for zoom view.
Comments
Post a Comment