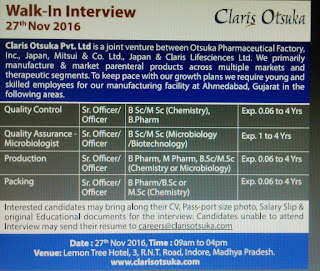
Claris Ostuka pvt Ltd is a joint venture between Ostuka Pharma company inc Japan & Claris life sciences. We primarily manufacture and market parental across multiple market and therapeutic segments. To keep pace keep our growth plan we require young and skilled employees for our manufacturing facility at Ahmedabad .
Date: 27th November 2016.
Time: 09 am to 04 pm
Must Check: Urgent Government JobsVenue: Lemon tree hotel , 3, RNT road , Indore, MadhyaPardesh
For more details click on below image for zoom view
Great Opportunity:
Dont Miss: MACLEODS Walk in Interviews
Tags: Claris ostuka career, Claris ostuka share price, Claris ostuka deal, Claris ostuka logo, Claris ostuka pvt ltdMust Check:
For Latest updates in Pharma Job in Govt and Pvt sector just Like our Facebook page: Pharma Education & Pharma jobs
Comments
Post a Comment